Two kinds of tests are available for coronavirus (COVID-19): virus tests [antigen tests, polymerase chain reaction (PCR)] and antibody tests.
- Virus tests to check if you have a current infection.
It checks for the presence of SARS-CoV-2, the virus that causes coronavirus, in your respiratory system. In most cases, results from point-of-care tests may be available at the testing site in less than an hour and others must be sent to a laboratory for analysis that can take a few days.
- Antibody tests to check if you had a past infection.
It may not show if you have a current coronavirus infection because it can take 1–3 weeks after infection for your body to make antibodies. In most cases, results must be taken from blood samples and sent to a lab for analysis.
For more information on the coronavirus, please visit the Centers for Disease Control and Prevention (CDC) and Texas Department of State Health Services website below.
CDC recommendations on how to test for coronavirus
To fight against the spread of coronavirus, TaiDoc dedicates in developing a rapid antigen test that can be used for the detection of SARS-CoV-2 in nasopharyngeal secretions.
TD-4531
VTRUST COVID-19 Antigen Rapid Test
- Fast results in 15 minutes
- Visual interpretation
- Two colored indicator on test cassette
| Test Principle | Lateral Flow Chromatographic Immunoassay |
| Target Antigen | SARS-CoV-2 Nucleocapsid Protein |
| Sample Type | Fresh Naspharyngeal Swab Specimen |
| Limit of Detection (LoD) | 1.26 x10^2TCID50 per mL |
| Storage condition | 2-30℃/36-86℉ |
| Cross-Reactivity & Interferences | Viruses, Bacteria and Interferences tested do not cross-react or interfere |
| Reaction Time | 15 minutes |
Clinical Performance
Comparator | ||||
POS | NEG | Subtotal |
||
VTRUST COVID-19 Antigen Rapid Test (TD-4531) | POS | 67 | 1 | 68 |
| NEG | 5 | 243 | 248 |
|
| Subtotal | 72 | 244 | 316 |
95% CI |
||
Sensitivity: Positive Percent Agreement(PPA) | 93.1% | 83.0% - 97.2% |
Specificity: Negative Percent Agreement (NPA) | 99.6% | 97.7% - 99.9% |
Positive Predictive Value (PPV) | 98.5% | 91.6% - 99.7% |
Negative Predictive Value (PPV) | 98.0% | 95.6% - 99.1% |
Overall Percent Agreement (OPA) | 98.1% | 95.9% - 99.1% |
Package Contents
20-Test Kit/Box
- Sterile Nasopharyngeal Swabs ×20
- Test Cassette ×20
- Extraction Tube ×20
- Extraction Tube Dripper ×20
- Extraction Buffer ×2
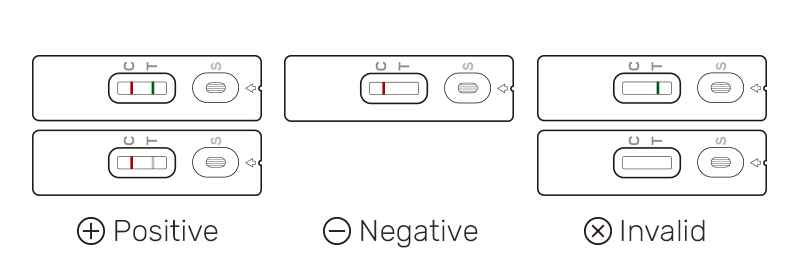
Interpretation of Results
| Result | Interpretation | |
| Antigen test | Positive | Most likely* you DO currently have an active COVID-19 infection. |
| Negative | Most likely* you DO NOT currently have an active COVID-19 infection. | |
| Antibody test | Positive | You likely* have HAD a COVID-19 infection. |
| Negative | You likely* NEVER HAD (or have not yet developed antibodies to) COVID-19 infection. | |
| BOTH(antigen and antibody test) | Antigen Positive, Antibody Positive | Most likely* you DO currently have an active COVID-19 infection |
| Antigen Positive, Antibody Negative | Most likely* you DO currently have an active COVID-19 infection | |
| Antigen Negative, Antibody Positive | You likely* have HAD and RECOVERED FROM a COVID-19 infection. | |
| Antigen Negative, Antibody Negative | You likely* have NEVER HAD a COVID-19 infection. |
*No test is ever perfect. All tests occasionally result in false positive or false negative results. Sometimes the results are not definitive. For this and other reasons, results should always be reviewed by healthcare professionals.
Note. Data for interpretation of results from CDC (2020).
For more information on the COVID–19, please visit the Centers for Disease Control and Prevention (CDC) website below.