Dear partners,
We are always thankful for our valued customers’ contributions and input in making sure that our company’s products and services fit your needs. We would not be in the position without your support and as such, we would like to thank for your continuing support.
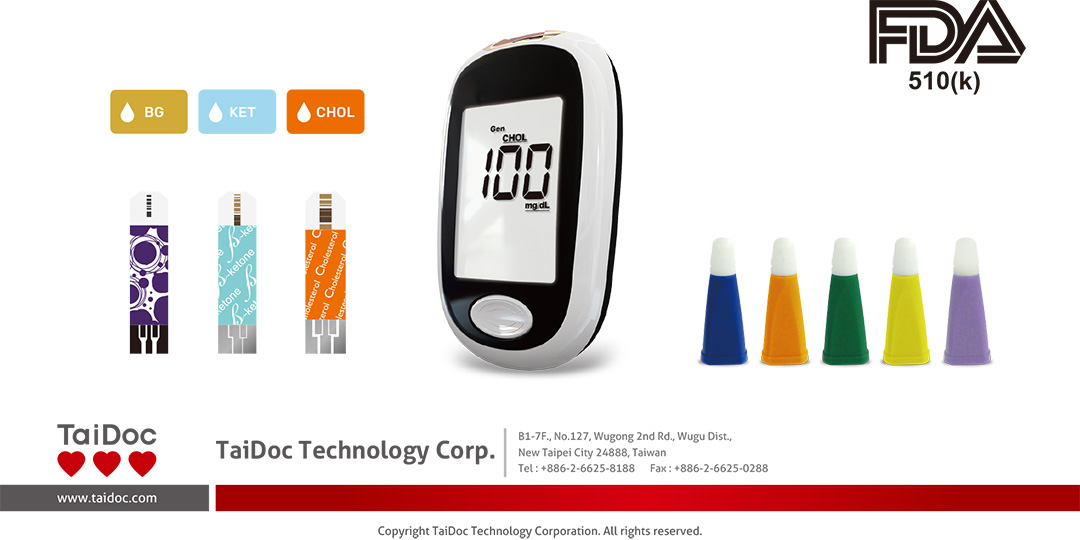
Based on the customer’s demands, and market trend that we have submitted cholesterol parameter to FDA and gratefully to announce that it has passed FDA Class II with 510(k) in June 2022.
We are the first one FDA certified with glucose, ketone and cholesterol parameters onto XPER Technology Procheck Advance Multi-Functional Monitoring System TD-4206 because the test strips have high stability and accuracy by coating with gold and laser engraving technologies.
It is also a priority to upgrade AKTIVSAFE LANCET (Sterile) TD-5052 for professional use as FDA Class II product with 510(k). We have completed this before FDA’s deadline in November in order to fulfill the demand in market.
We hope you will be pleased with this news and look forward to receiving your feedback and response.
Thank you!
Sincerely,
TaiDoc Technology Corp.